A Gentler Strategy Against Childhood Tooth Decay
The Problem: Early Childhood Caries
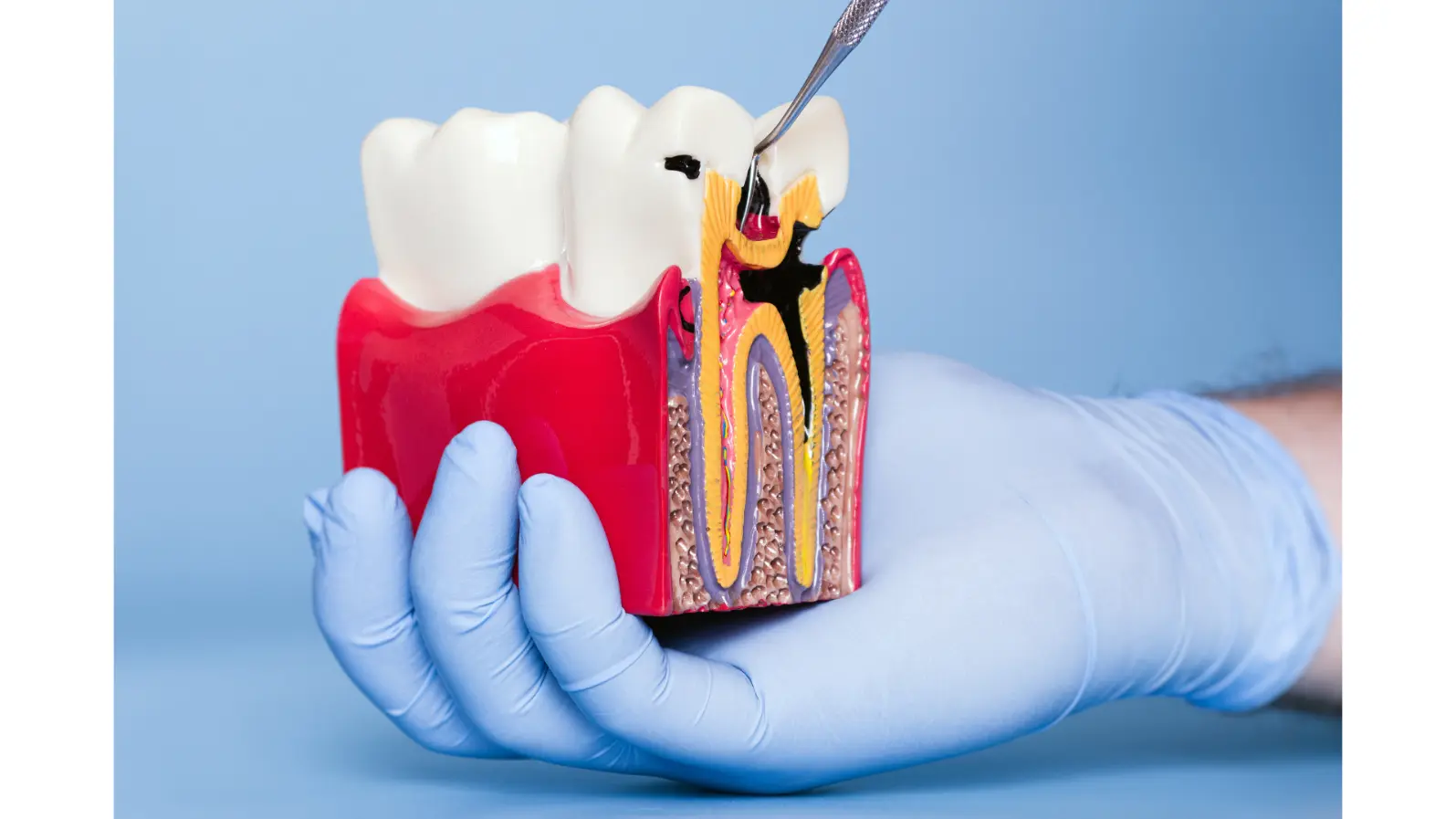
Early childhood caries (ECC) — a severe, fast-spreading form of tooth decay — remains one of the most stubborn oral health issues worldwide. It tends to strike young children, eroding their teeth quickly and often leading to pain, infection, and costly dental treatments.
Scientists have long known that ECC isn’t caused by a single microbe. Instead, it results from cross-kingdom cooperation between bacteria and fungi — specifically, Streptococcus mutans (a cariogenic bacterium) and Candida albicans (a common fungus). Together, they build sticky, acid-producing biofilms that cling stubbornly to teeth and dissolve enamel.
A New Approach: Breaking the Biofilm Bond
Instead of trying to kill these microbes — which can disrupt the healthy oral microbiome and cause drug resistance — researchers at the University of Pennsylvania tried a more precise, non-toxic strategy.
Their idea: disrupt the partnership between S. mutans and C. albicans at its molecular handshake.
The team identified a key interaction point: an enzyme from S. mutans called glucosyltransferase B (GtfB) binds to mannans, a type of sugar molecule on the C. albicans cell wall. This bond helps the two species stick together and form virulent biofilms.
To break this link, the researchers used mannan-degrading enzymes (MDEs) — biological “scissors” that cut apart mannans and prevent GtfB from attaching.
How They Tested It
The team experimented with three enzymes:
α-mannosidase
β-mannosidase
β-mannanase
They grew mixed biofilms of S. mutans and C. albicans on surfaces coated with human saliva (to mimic real oral conditions). Using advanced imaging, biochemical tests, and atomic force microscopy, they measured how the enzymes affected biofilm formation, acidity, and tooth enamel damage.
The Results: Big Impact, No Killing
The results were striking:
Biofilm biomass dropped sharply, especially with β-mannanase (up to 2.5× reduction).
Acidity decreased — the pH rose above the critical level that causes enamel erosion.
Binding forces between S. mutans and C. albicans weakened dramatically — by about 15-fold.
Enamel surfaces stayed intact, showing far less demineralization.
Importantly, the enzymes were non-toxic to human gingival cells and did not kill the microbes, meaning they preserved the natural oral ecosystem.
Why It Matters
This research represents a new paradigm in fighting oral disease — one that focuses on disrupting harmful microbial relationships rather than killing microbes outright. Such precision targeting could avoid side effects like microbiome imbalance or antibiotic resistance.
By weakening the cooperative bonds in these “cross-kingdom” biofilms, therapies based on mannan-degrading enzymes could help prevent tooth decay in a safer, more sustainable way — especially in children prone to ECC.
Reference
Kim H.E., Dhall A., Liu Y., Bawazir M., Koo H., & Hwang G. (2021). Intervening in Symbiotic Cross-Kingdom Biofilm Interactions: A Binding Mechanism-Based Nonmicrobicidal Approach. mBio, 12(3): e00651-21.
https://doi.org/10.1128/mBio.00651-21